
Ark Biopharmaceutical Secures China Marketing Approval for Aizhida, Expanding Treatment Options for ADHD
NMPA approval of Aizhida marks a milestone in pediatric and adolescent mental health care, addressing significant unmet needs in China’s ADHD treatment landscape
Shanghai: Shanghai Ark Biopharmaceutical Co., Ltd. (ArkBio) has announced that China’s National Medical Products Administration (NMPA) has granted marketing authorization for Aizhida, a novel treatment for Attention Deficit Hyperactivity Disorder (ADHD) in patients aged six years and older. The approval follows the successful review of the New Drug Application for Serdexmethylphenidate Chloride and Dexmethylphenidate Hydrochloride Capsules, marking a significant advancement in ADHD therapeutics in China.
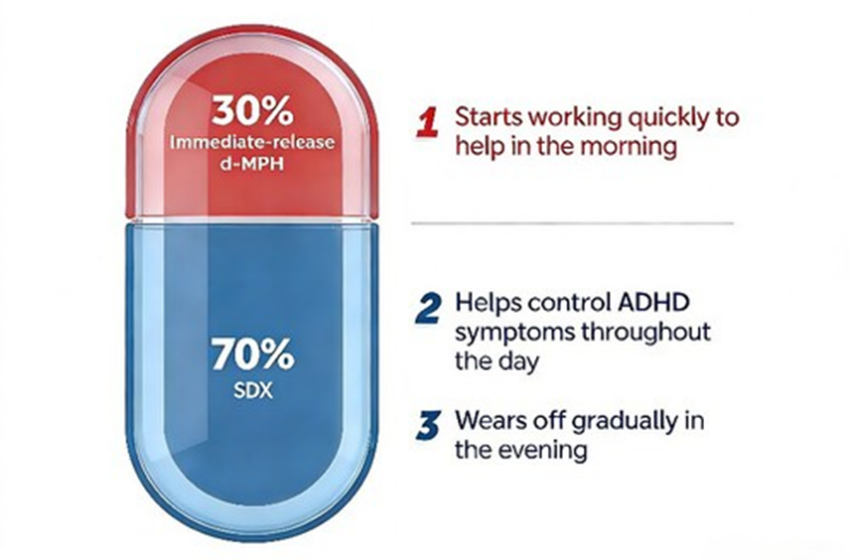
Aizhida is a central nervous system stimulant designed as a once-daily oral capsule that combines two complementary mechanisms of action. Each capsule contains 70% serdexmethylphenidate (SDX)—a prodrug of dexmethylphenidate—and 30% immediate-release dexmethylphenidate (d-MPH). This fixed-ratio formulation is engineered to deliver both rapid symptom relief and sustained therapeutic coverage throughout the day. Following ingestion, the immediate-release component provides an early onset of effect, while SDX is gradually converted into active d-MPH in the lower gastrointestinal tract, ensuring extended symptom control.
The drug’s approval in China builds on its prior regulatory success in the United States, where Aizhida was approved by the U.S. Food and Drug Administration in March 2021 as a once-daily treatment option for ADHD patients aged six and above. Its entry into the Chinese market represents an important step in broadening access to innovative ADHD therapies in regions where treatment choices remain limited.
ADHD is a chronic neurodevelopmental disorder that typically begins in childhood and often persists into adolescence and adulthood. It is characterized by persistent patterns of inattention, hyperactivity, and impulsivity that can significantly impair academic performance, social functioning, and quality of life. In China, ADHD prevalence among children and adolescents is estimated at approximately 6.4%, affecting more than 23 million individuals. Clinical studies indicate that symptoms continue into adolescence in 60–80% of cases and into adulthood in nearly half of patients.
Despite the high prevalence, treatment options for ADHD in China have been constrained, with existing medications not fully meeting clinical needs related to efficacy, safety, and dosing convenience. Many patients discontinue therapy due to inadequate symptom control or adverse effects, highlighting the demand for more effective and patient-friendly alternatives.
Aizhida’s approval is supported by robust clinical evidence. In a pivotal Phase III clinical trial conducted in Chinese patients, the drug met both its primary and key secondary endpoints. The study demonstrated statistically significant and clinically meaningful improvements in core ADHD symptoms compared with placebo across all assessment time points. With historical supply limitations for traditional single-agent therapies, Aizhida’s approval introduces a valuable new option for clinicians and patients alike.
Experts have highlighted the significance of this development. Professor Yi Zheng, Chief Expert at Beijing Anding Hospital and Lead Principal Investigator of the Phase III trial, noted that the approval of Aizhida offers clinicians a much-needed new therapeutic tool, particularly for patients who respond poorly or experience intolerance to existing treatments. Professor Jing Liu, Director of the Child Psychiatry Center at Peking University Sixth Hospital, emphasized that expanding pharmacological options is essential for advancing standardized ADHD care and improving long-term outcomes.
The dual-action design of Aizhida—delivering rapid onset with all-day symptom control—positions it to potentially reshape the ADHD treatment paradigm in China. As the first medication in the country to combine these attributes in a single formulation, it fills a critical clinical gap.
About ArkBio
Founded in 2014, ArkBio is a global biotechnology company focused on developing innovative therapies for respiratory and pediatric diseases. The company has built a differentiated research and development pipeline through in-house innovation and strategic collaborations. Its key assets include ziresovir (AK0529) for RSV and AK3280 for idiopathic pulmonary fibrosis, alongside Aizhida (AK0901), which has now entered the commercial launch phase in China.